✍️ Author: Dr Eleni Christoforidou
Home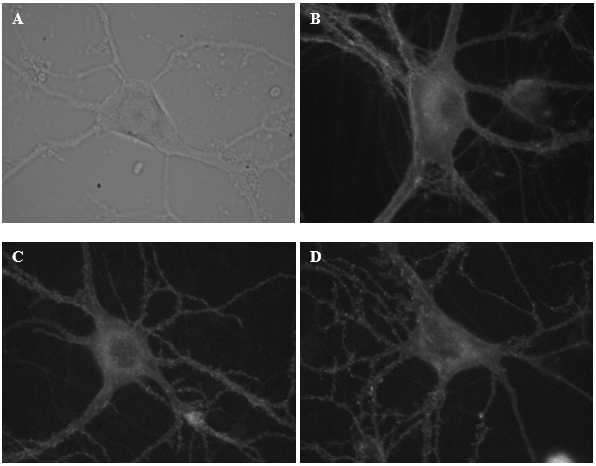
These are some of the neurons from which I recorded electrical activity today. Image (A) illustrates a neuron under a DIC microscope. Images B-D display neurons labelled with a fluorescent reporter.
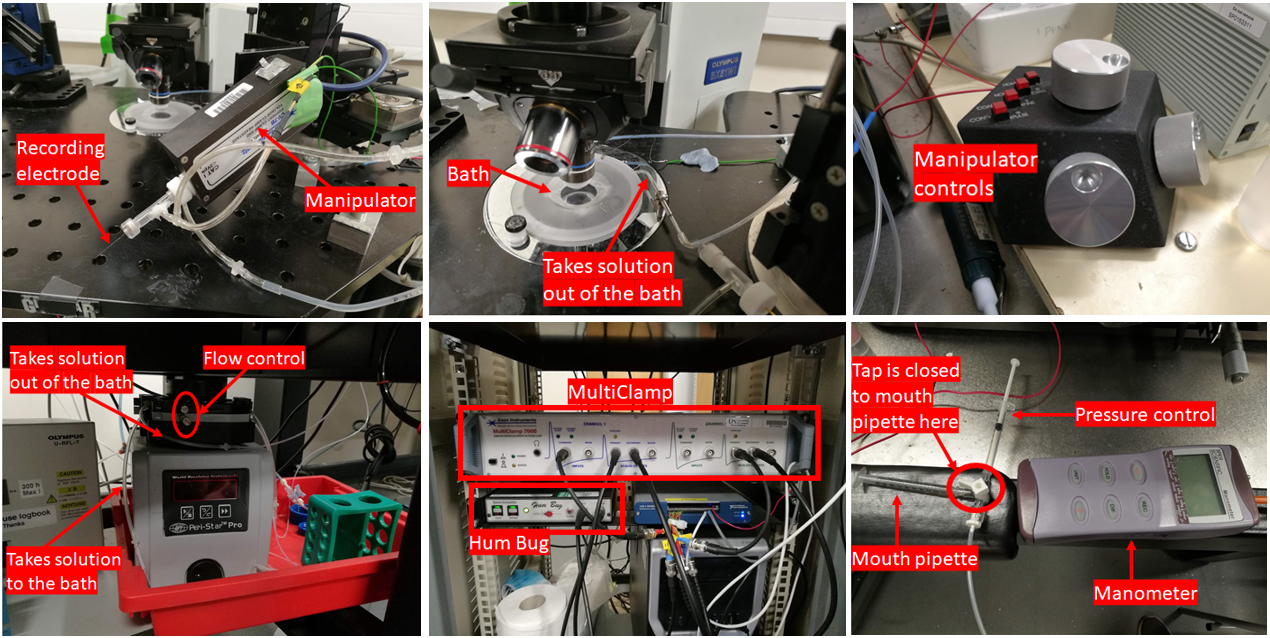
The array of equipment employed for my experiment.
A Day in the Lab: Unravelling Neuronal Activity using Electrophysiology
🕒 Approximate reading time: 4 minutes
Today's adventure in the lab revolved around an electrophysiology experiment, with a particular emphasis on the patch-clamp technique. The underlying concept involves utilising an electrode to record the electrical activity of a solitary neuron grown in vitro. Admittedly, the procedure can initially seem somewhat challenging, but with practice, it becomes significantly more manageable. Over the past two months, I have successfully recorded activity from roughly 100 individual neurons, each requiring approximately 20 minutes of dedicated attention.
Presented below are images that depict what the neurons appeared like under the microscope. Further details on the experiment and the methods I employed are detailed subsequently.

To comprehend how neuronal activity is recorded and how to operate the recording equipment, one requires a solid understanding of physics and electronics. Electrophysiology demands it. Below, I've included an image of the various recording equipment utilised in today's experiment. The major components are labelled for the convenience of the curious among you. The setup principally involves a DIC (differential interference contrast) microscope connected to a range of electronic devices.

Delving into the Methodology of Today's Experiment
The recordings were performed employing a computer-controlled patch clamp amplifier, which was operated with specialised computer software. Whole-cell voltage clamp recordings were performed at a holding potential of -65 mV at room temperature. The whole-cell configuration was achieved by applying a gentle suction pulse orally while delivering a brief 1 V electrical pulse simultaneously. Compensation was applied for the fast and slow capacity transients. A seal test (a 10 mV test pulse) was administered through the recording electrode to measure the access resistance at the commencement of every 10-second recording.